- Material Science Insights
- Silicon 101 - What is silicon used for? (3/3)
Silicon 101 - What is silicon used for? (3/3)
Many sources tend to mix up applications of silicon and the materials that contain it – a matter that is complicated further by the fact that they’re all ultimately produced from naturally-occurring silica (or silicates). So to adopt a more logical approach, we’ll talk first about the applications of elemental silicon, and then go on to mention the uses of silica and silicates, followed by the chemicals derived from them.
The applications of silicon
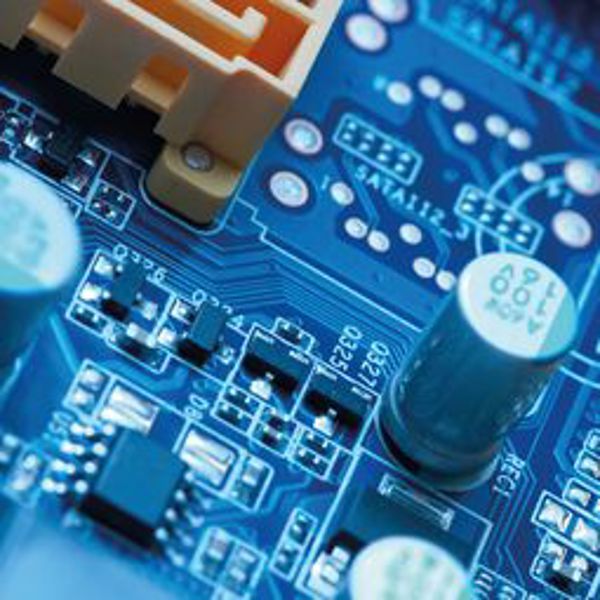
Arguably the most important application of silicon is as the physical support for integrated circuits. Formed from a wafer of monocrystalline silicon, the parts that need to act as circuits are doped, and these circuits are insulated from each other by a layer of silicon dioxide. Because modern integrated circuits are so small (with >100 million transistors per mm2), highly pure monocrystalline silicon is needed – at such small scales any impurities or deviation from perfect crystallinity would disrupt the electrical properties.
The other major application is in solar cells, where silicon performs the essential role of converting the energy from incident light into electrical energy. Monocrystalline solar cells have the highest energy-efficiency, and so have long been the favoured option – although there has sometimes been competition from cheaper grades of multicrystalline silicon. Amorphous silicon is also used in solar cells, although its low efficiency means that applications are restricted to low-power applications, or those where its ability to form thin coatings is useful.
A recent exciting application for silicon is as a replacement for graphite in the anodes of lithium-ion batteries, for which it offers considerably greater lithium ion capacity and so greater charge storage potential. Up until recently, expansion/contraction issues upon lithiation and delithiation made using silicon impractical, but much work is currently being done to overcome these challenges, and so open the door to a new generation of high-power batteries.
Metallurgical-grade silicon also has a major use in the production of aluminium–silicon alloys, which are used in castings (largely for engine parts because of their light weight and high strength).
Last but not least (at least for us and our customers!), silicon is used in a number of specialized industrial applications. These include:
- As an alloying element (with copper) in aluminium joints, which allow them to be brazed (welded) at relatively low temperatures.
- As a fine powder for plasma spraying on sputtering targets.
- In pyrotechnic ignition compositions, for example as a delay charge.
The applications of silica and silicates
In contrast to elemental silicon, which has only found applications in the last 70 years or so, silica and silicates have been fundamental to human civilization for many thousands of years. This is a result of their natural abundance, stability, and almost infinitely variable structural formulae, leading to a vast array of physical properties.
Some of the applications of silica (and the form in which it’s used) include:
- Concrete, refractories, oil & gas and mortar production (silica sand, silica fume)
- Ferroalloy production (quartz or quartzite)
- Glass production (silica sand)
- Toothpaste abrasive (hydrated silica)
- Optical fibers (fused silica)
- Crystal oscillators (quartz)
- Semiconductor passivation (direct silicon oxidation)
- Fining agent in beverages (colloidal silica)
- Anti-caking agent in food (colloidal, precipitated or fumed silica)
- Drying agent (silica gel)
- Car tire filler (amorphous silica)
- Insulation (silica sand)
- Filtration medium (kieselguhr).

Some of the applications of silicates (and the specific minerals involved) include:
- Portland cement (calcium silicates)
- Clay-derived ceramics (e.g. kaolinite)
- Ceramic glazes (feldspar)
- Refractory materials (various silicates)
- Abrasives (garnet)
- Molecular sieves and catalysts (zeolites)
- Silicate paints (potassium silicate)
- Dental glass ionomer cements (feldspar)
- Waste water flocculation/coagulation (sodium silicates)
- Dye fixatives (sodium silicates)
- Horticultural growing media (vermiculite mica)
- Gemstones (e.g. garnets, opal, jade).
The applications of other silicon compounds

One of the most significant uses of silicon/silica is in the production of industrial alloys and commodity chemicals, of which these are the most important:
- Ferrosilicon (FeSi) is made by heating silica, carbon and iron ore (or steel), and is used as a deoxidation and alloying agent in steel manufacture. Because this is such a large market, more silicon is produced in the form of ferrosilicon than as pure silicon!
- Silicon carbide (SiC) is made by heating silica (or silicon) and carbon, and is used as an abrasive, and also in high-temperature semiconductor electronics and ceramics.
- Silicon nitride (Si3N4) is made by heating silicon and nitrogen, and is used as an abrasive and in ceramics.
- Ferrosilicon nitride (FeSi3N4) is made by the action of nitrogen upon ferrosilicon at high temperature, and is largely used in taphole clay in blast furnaces.
- Organosilyl chlorides (SiRnCl4–n) are usually formed by the action of alkyl halides upon silicon in the presence of copper, and have value as synthetic intermediates.
- Siloxanes ((HO–(SiR2–O–)nH) are short-chain molecules largely formed by the hydrolysis of organosilyl chlorides. The linear versions are used in some ceramics, while the cyclic equivalents are used in cosmetics.
- Silicones ((–SiR2–O–)n) are polymers, made by a variety of methods, which are commonly used in antifoaming agents, rubbers, caulks, sealants, adhesives and coatings.