- Magazine
- Trainee Blog
- Graphite, lithium-ion batteries and hiking in Kristiansand
Graphite, lithium-ion batteries and hiking in Kristiansand
Approximately nine months ago I started as a Trainee in Elkem and a lot has happened since my first day, from meeting the other trainees at the trainee gathering to taking part in developing the product in Elkem’s new investment on battery graphite.
My journey started at the trainee gathering at Kalvåg where I got a nice introduction to Elkem and the trainee program, in addition to getting to know the other trainees. It was very valuable to meet the second-year trainees, which have a lot of experience and useful advice for us new trainees. During the trainee gathering we visited Elkem Bremanger, where we got a tour on the production site to see the different furnaces and the demolished furnace 5 from the Hornelen project. It was really exciting to see the inside of a smelting furnace! Apart from enjoying wonderful seafood, the afternoons were mostly spent on hiking trips in beautiful surroundings with great company.
 Figure 1: The first-year trainees at the Elkem Headquarters in Oslo. From left: Mia, Mina, Lene and Egil. (Photo credit: Otto Evenstad)
Figure 1: The first-year trainees at the Elkem Headquarters in Oslo. From left: Mia, Mina, Lene and Egil. (Photo credit: Otto Evenstad)
After the trainee gathering, I moved to Kristiansand to start my first trainee period working for Elkem Battery Materials in the Carbon Division at Fiskaa. Graphite is the dominating anode material in lithium-ion batteries and the need for battery graphite will increase with increasing demand for electric vehicles and other electronics.
In June 2019, Elkem decided to invest 65 million NOK in the construction of an industrial pilot facility for production of anode graphite for lithium-ion batteries in Kristiansand. This is an important step towards the goal to build a large-scale industrial plant for production of battery graphite which is more energy efficient and has a greener footprint than the traditional graphite production methods.
One of the measures made towards a more environmentally friendly graphite production is the use of hydropower driven closed furnaces for the graphitization step. The most common method used for graphitization today is heating in an Acheson furnace. However, this method is less energy efficient and has a higher degree of emissions compared to the closed furnaced used by Elkem. In addition, most of the battery graphite producers today are located in Asia. Hence, a battery graphite production site in Europe is desired as this reduces transport emissions to battery producers in Europe.
I feel very lucky to spend my first trainee period in the Elkem Battery Materials project. I have a background in Nanotechnology from NTNU with a specialization within materials, energy and environment, and it was natural for me to take part in the product development. My mentor was Gunstein Skomedal, who once was an Elkem trainee himself, and he made sure I got a nice introduction to the project by letting me participate in all the process steps from raw material to final product. The battery graphite is generally made by calcining coke, which is then milled and shaped to obtain the desired particle size (around 15-20 µm). The next step is graphitization, which is done in a special closed furnace at very high temperatures, followed by coating before the final coin cell testing step.


Figure 2: To the left: The battery graphite is a black and very fine powder which is mixed with other components to create a slurry. To the right: Graphite slurry being coated onto a Copper foil. The coated Copper is then dried and ready for lab scale testing in coin cells. (Photo credit: Elkem)
Most of my time at Elkem Battery Materials has been dedicated to two projects, one about wettability and one on image analysis. I have been working on developing a method for testing the wettability of the battery graphite. Control of graphite anode wettability is important to be able to fine tune the product properties. The battery electrolyte should have a high degree of wettability on the graphite anode, which means that it should spread easily onto the anode and enter all pores in the structure. The more graphite in contact with the electrolyte, the better the battery performance.
In addition to wettability, I have developed a method for analysing graphite particle shape by Scanning Electron Microscopy image analysis. This work was done together with Bente Kroka from the Materials Characterization Group at Elkem Technology. Information about the particle shape, together with results from other characterization techniques, makes it possible to predict the behaviour of the graphite powder such as powder flow and packing. If there is something I have learned from my time working in the battery materials group, it is that graphite is not only graphite. There are amazingly many parameters one can fine tune to obtain completely different product properties. Every detail matters!
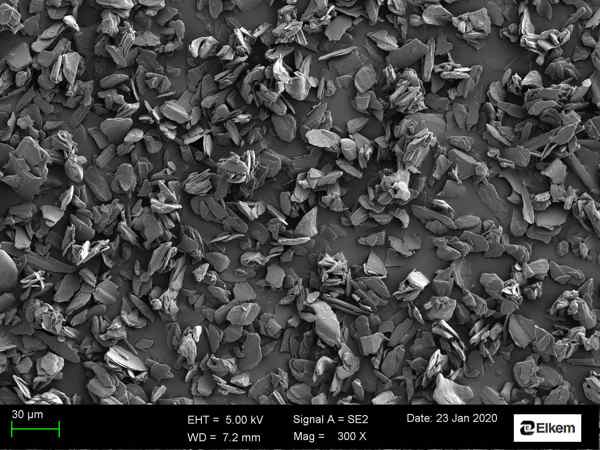
Figure 3: Graphite particles produced by Elkem Battery Materials which has been imaged using a Scanning Electron Microscope (SEM). (Photo credit: Bente Kroka)
Apart from graphite, my days in Kristiansand have been filled with social activities and hiking trips. There are many former trainees living in Kristiansand that are very eager to arrange social gatherings with board games and dinners. In Kristiansand they have something called “Terrengkarusellen”, a running competition every week located at different parts of the city and the nearby woods. This is a perfect way of getting to know Kristiansand if you like running. I even participated in a run through Dyreparken! Kristiansand also offers a lot of cultural activities at Kilden, where you can attend a concert with the Symphony Orchestra or listen to the Kilden Choir.

Figure 4: The view from the top of “Den omvendte båt”, a highly recommended hiking destination in Kristiansand.

Figure 5: Fiskåvannet viewed from the top of Øyliheia in the woods nearby Vågsbygd, Kristiansand. (Photo credit: Margrethe Grønn)
Sadly, my time in Kristiansand is approaching its end for now. I would like to thank all my colleagues in the Elkem Battery Materials group for the warm welcome and for making me a part of the team. I also want to thank everyone I have had the pleasure to collaborate with during my work. I have learned a lot from cooperating with colleagues across departments and divisions, and everyone has been very welcoming and eager to help. Now, new adventures await at Elkem Bjølvefossen where I will spend my second trainee period learning about smelting furnaces and the FSM process.