- Elkem’s recent contribution to the development and innovation of foundry products
Elkem’s recent contribution to the development and innovation of foundry products
Leander Michels is one of Elkem’s Research Scientists in Kristiansand. Before moving to Norway to pursue a PhD in Physics, Leander worked as a high school teacher in Brazil, where he was born. After a few years as a post-doc, he was hired by the Elkem Foundry Innovation team to support the internal knowledge and to increase Elkem advantage in the knowledge gap against competitors.
This week, we sat down with Leander to discuss his recent publication “Elucidating the chemical interactions and turbostratic structures in spheroidal graphite cast irons” in one of the most prestigious journals, Carbon. If you have the academic background to understand the science, I highly recommend you read the article. But if you, like me, had a hard time with the title, I suggest you keep reading to learn more about the important implications of this work.
Background
Cast iron is an alloy containing iron, silicon, and carbon used in many sectors, such as wind energy, engineering, and automotive industries. Many of Elkem's foundry products, particularly the inoculants and preconditioners, are designed to promote the formation (nucleation) of graphite crystals in cast iron. In fact, inoculants are vital for avoiding metallurgical defects in cast iron caused by insufficient nucleation. In simple terms: the more graphite formation Elkem’s products can prompt, the better.
The nature of this graphite formation is still puzzling, and several explanations are circulating. However, it is mostly accepted that Elkem’s products specifically promote the formation of micro-inclusions, which act as a starting point (nucleation site) for the graphite to form. This is supported by recent advances in electron microscopes. Yet, the question remains: Why does graphite ‘like’ some of these micro-inclusions? There have been many attempts to answer this phenomenon.
A large European customer of Elkem prompted the work to answer this question. A manager studying the mechanism of how one of Elkem’s products worked in his foundry production process had conducted several trials and evaluated the results for years without success. As a result, Elkem was contacted, and Leander sat down with the customer to see how they could address the issue together. However, after starting the work and discussing the results, the ambition grew. It evolved from explaining a simple mechanism to exploring an entirely different view of how the nucleation of graphite works.
The study
In the article, Leander and the co-authors, the polish researchers Dr. Bogdan Cygan, Prof. Miroslawa Pawlyta and Prof. Jan Jezierski from Silesian University of Technology in Poland, the fellow Elkem scientist Adam Götz and Prof. Jaakko Akola from Norwegian University Science and Technology (NTNU), studied the graphite formation on a nitride micro-inclusion. These (Al, Mg, Si)-nitrides, Mg-sulfides and Mg-oxides, and others have historically been found inside graphite nodules; but as mentioned, foundrymen and scientists struggle to explain why they act as nucleation sites. In materials science and metallurgy, the explanation was typically based on previous work from Bramfitt (1973) and Turnbull & Vonnegut (1952).
“You can think of this as a lego model,” Leander explains when asked to describe the theory in layman's terms. “The models say that the nucleation sites [such as (AI, Mg, Si)-nitrides] are ‘good’ if they are from the same type of lego as graphite (crystallographic match)” he describes further. “Lego from the same brand can easily be attached. Also, if the nucleation site has a different brand from the graphite, they can still attach, but you will need to use brute force (higher undercooling)” Leander says.
To understand the authors’ key theory supporting their findings, Leander explains: “These models work well for solidification of metals, but carbon is not a metal”. In other words, carbon and graphite do not need to behave in the way metals do. “People haven’t considered that carbon likes to form chemical connections, and when you apply chemistry to this, it becomes an entirely different universe,” Leander says, pointing to their findings.
Findings
“We showed that the chemical bonding [not the ‘lego theory’ patterns] is the main form of attachment,” Leander shares. The sites of the nitride with Al and Si on the surface are the locations where the chemical bonding occurs, as seen in the figure below. In the article, the authors also provide a comprehensive description of how graphite grows in the cast iron alloys.
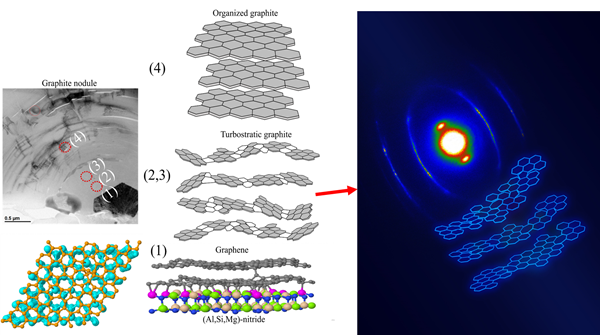
Practical implications
These findings are directly related to Elkem’s foundry business, specifically considering the optimization and innovation of new products. For example, in finding replacements for critical materials, these findings will significantly contribute to the work of identifying and evaluating potential substitutes.
Moreover, this work is not just important for Elkem’s organization. As previously stated, this work was carried out in close collaboration with a customer. Customers may not have the capacity to delve into such matters. As such, research from partners like Elkem is extremely valuable to increase our customers' understanding while lending scientific credibility and generating significant interest in Elkem’s brand.
From Elkem, this work had the scientific support of Cathrine Hartung, Emmanuelle Ott from the Foundry Innovation, and Alberto Giacomoni from the Foundry Technical Customer Service.
The article can be downloaded as an open access due to an agreement with the Norwegian University of Science and Technology (NTNU), where Leander Michels is an affiliated researcher: ScienceDirect