- Magazine
- Material science insights
- Silicon 101 - The properties of silicon (2/3)
Silicon 101 - The properties of silicon (2/3)
Silicon is a metalloid, meaning its properties fall between those of a metal and a non-metal. The properties of the two allotropes vary somewhat, but as most studies have been done on crystalline silicon, that’s what we’ll focus on.
The physical properties of silicon
Silicon, with its strong, diamond-like tetrahedral structure, is quite resistant to heat – the melting point is 1414 °C, while the boiling point is 3265 °C. The same relatively open network also lies behind its low density of only 2.33 g/cm3, and the fact that (like water), it becomes more dense when it melts, with a density of 2.52 g/cm3 at its melting point.
The rigid tetrahedral structure also explains its hardness (7 on the Mohs scale, the same as quartz) and its high ultimate tensile strength (about 100–200 MPa). But the inability of defects to move within the structure means that silicon has a low toughness, and will shatter like glass at room temperature – although as the temperature rises beyond 540 °C it very quickly becomes ductile.
Speaking of temperature, the thermal conductivity of pure silicon is quite high at 150 W/(m·K), behind only a handful of the most conductive metals (and diamond). This might be surprising given that it doesn’t have many conduction electrons, which are the main reason for the high thermal conductivity of metals. But it becomes understandable when we consider the rigid, highly ordered structure of silicon, and its consequent ability to transmit heat by high-energy lattice vibrations (phonons) with minimal scattering.
The electrical properties of silicon
In contrast to thermal conductivity, when we’re talking about electrical conductivity, the small number of conduction electrons in pure silicon means that it is closer to being an insulator than a conductor, with a conductivity of just 1000 S/m, in contrast to most metals, which fall in the range 106–107 S/m.
However, silicon has the property that electrons and holes produced by thermal excitation can move through the lattice under the application of a voltage. This results in the conductivity of silicon increasing at higher temperatures, unlike metals, which exhibit decreasing conductivity with temperature. This characteristic makes pure silicon an intrinsic semiconductor.
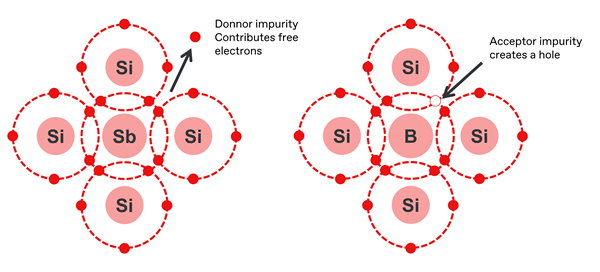
The currents able to be sustained by pure silicon are relatively low – but all that changes when atoms of silicon are replaced with other elements, in a process known as doping. This greatly increases the number of charge carriers available, and the conductivity increases dramatically. There are two types of doped silicon:
- Dopants with three valence electrons, such as boron, aluminium, indium and gallium, produce a local ‘hole’ or area of positive charge, and result in a p-type semiconductor.
- Dopants with five valence electrons, such as phosphorus, antimony and arsenic, contribute an extra electron, producing a local negative charge, and result in an n-type semiconductor.
Joining the two types of doped material together produces a p–n junction, which has wide application in electronic devices.
Silicon 101 - What is silicon used for?
The chemical properties of silicon
Before moving on to other things, it’s worth spending a moment on the chemistry of silicon.
Because of its filled valence shell when in bulk, and the presence of an atomic layer of oxygen on the surface, the reactivity of the element itself is not high at room temperature, with silicon being attacked only by gaseous fluorine and by hydrofluoric acid. At higher temperatures, however, it dissolves readily in hot alkali, and reacts with gaseous non-metals (including the other halogens) in the range 300–1000°C.
The first ionization energy of silicon (the energy required to remove an electron from the uncharged atom) is not especially low (787 kJ/mol). Combined with its middling electronegativity (1.90) – the ability to attract electrons towards itself – this means that its bonds are always covalent rather than ionic in character.

Of the bonds formed by silicon, the one with oxygen is significant for being very strong (452 kJ/mol), making it a driving force in much of the chemistry of silicon. The result of this interaction between silicon and oxygen, of course, is the familiar silica (quartz or SiO2) and its various morphological and color forms such as chalcedony, amethyst and citrine. Also important geologically are an array of complex rock-forming silicates, which together with silica make up more than 90% of the earth’s crust.