- Magazine
- Material science insights
- Silicon 101 - Introduction to silicon (1/3)
Silicon 101: Introduction to silicon (1/3)
Silicon, when crystalline and ultra-pure, is a blue-grey, crystalline solid with a glossy, metallic sheen. First isolated in 1824 by Berzelius, silicon has atomic number 14, and is a metalloid with semiconducting properties.
Introducing silicon
If all you associate with silicon are integrated circuits and solar cells, you’re not alone: these applications of element number 14 have certainly attracted the bulk of the attention over the past few decades. But important and widespread as these applications are, there’s much more that’s worth knowing about silicon.

When was silicon isolated?
Given that silicon is such an abundant element on Earth, its isolation as a pure element was far from straightforward. In 1789, Antoine Lavoisier had proposed that quartz (which we’re familiar with as silicon dioxide) was likely to be a compound of oxygen with an as-yet undiscovered but very common element. Unfortunately, his work was brought to a halt by the French Revolution, meaning that there was a delay before anyone was able to make further progress.
So it was not until 1811 that silicon was first isolated, when Joseph Gay Lussac and Louis Jacques Thénard treated silicon tetrachloride with potassium metal. From their description, the material they obtained must have been very impure, although oddly they didn’t make efforts to purify or study it further.
Instead, it was left to Jöns Jacob Berzelius to take the final step to prepare the pure element. This he did in 1824, by heating potassium fluorosilicate with potassium, giving a crude form of amorphous silicon, which he was able to purify simply by treating it with water.
Berzelius gave his new element the name silicium – from the Latin silex or silicis meaning flint. But seven years later in 1831, Thomas Thomson put forward the view that the element’s lack of metallic properties meant that it was better considered a non-metal. He therefore proposed that the ending should be changed to -on, to match the non-metals boron and carbon, giving the English name silicon that we use today.
What are the allotropes of silicon?
The amorphous silicon mentioned above, also known as a-silicon, is one of two allotropes of silicon, and takes the form of a brown powder. The exact structure of this material has always been elusive, but the latest thinking is that although there is no long-range order, the vast majority of the atoms are bonded to four others in a tetrahedral arrangement, albeit with a small percentage of three- or five-bonded atoms (‘coordination defects’).
The other allotrope, now known as crystalline silicon or c-silicon, was discovered later, in 1854, when Henri Deville, having accidentally produced aluminium silicide (Al4Si3), treated it with water to yield shiny platelets of crystalline silicon. With each atom tetrahedrally coordinated, the local structure of crystalline silicon has similarities to amorphous silicon, but the perfect ‘diamond cubic’ lattice significantly enhances its properties.
Two other terms in common use are worth clarifying here. Polycrystalline silicon (or ‘polysilicon’) contains randomly oriented crystallites of crystalline silicon that are typically 0.1–1 µm in size, while multicrystalline silicon usually refers to cases where the crystals are larger than 1 mm.
Silicon’s place on the periodic table
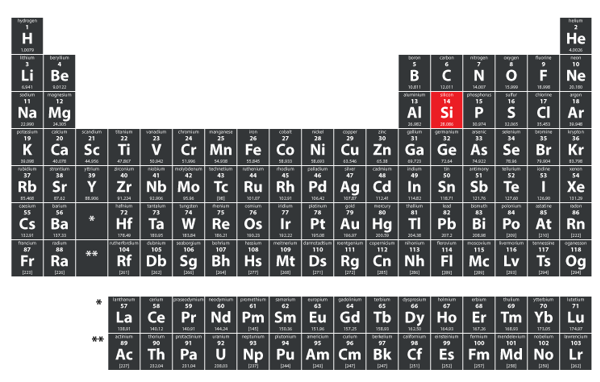
Silicon has atomic number 14, meaning that it has 14 protons and 14 electrons, with the most common isotope 28Si therefore containing 14 neutrons. Placed in the ‘p-block’ of the periodic table, it sits in row 3, between aluminium and phosphorus, and in group 14, being neighbored by carbon and germanium. Like these, it has four valence electrons, which has major implications for its bonding patterns.
The main isotope, 28Si, is stable and has a natural isotopic abundance of just over 92%, with the remaining 8% made up from two lesser-known stable isotopes, namely 29Si (which has minor applications in material sciences) and 30Si. These are sufficient to nudge the relative atomic mass of naturally-occurring silicon up to 28.0855. The most long-lived of the radioactive isotopes of silicon, 32Si, has a half-life of 172 years, and is used as an environmental tracer and for radiodating.
How was silicon formed?
Like the vast majority of heavier elements, virtually all the silicon we see around us today was formed billions of years ago in the cores of very massive stars that had used up their lighter elements, when their temperature had risen sufficiently to fuse together heavier nuclei.
In fact, the nucleus of the commonest isotope 28Si is a key stepping stone in that process, being formed at a temperature of about 2 × 109 K from the fusion of two 16O nuclei during the so-called oxygen-burning process (along with the release of a helium nucleus). Recent research suggests that silica itself is also formed in supernovae, explaining its widespread presence in cosmic dust and meteorites.
The abundance of silicon
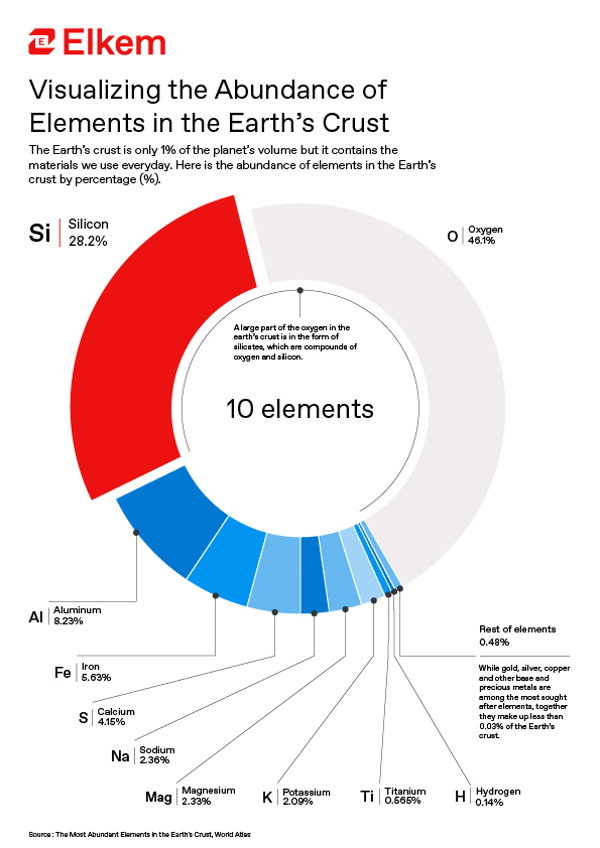
Perhaps then it is no coincidence that the vast majority of this silicon is bound up with oxygen, in the form of silica (quartz or SiO2) or one of the hundreds of known silicate minerals, which comprise SiO42– tetrahedra interspersed with metal cations.